Diamonds Aren't Formed from Coal. Here's How It Actually Happens

If you're a completist shopper like us, you always want to know where your products originate. You may know that With Clarity diamonds are ethically sourced and mined, but have you ever wondered where diamonds come from, period? As in, how are diamonds formed? What magic dust and dinosaur breath combined a million years ago to create the foundation of the modern engagement ring? Read on for a brief history — and future view — of diamond formation, and some fascinating facts about diamond strength and structure.
TABLE OF CONTENTS
Are Diamonds Made from Coal?
So diamonds were once pieces of coal that have been transformed under high pressure and temperature, right? Nope! This is an old wives' tale, just like "another drink will cure your hangover" or the idea that being out in the cold causes you to catch a cold. Diamonds are actually much older than plants, which are the main ingredient for the formation of coal.
The basic old-fashioned recipe for a diamond calls for:
- Carbon deposits, deep within the earth, that are subjected to temperature and pressure.
The exact time that it takes for a diamond to form within the earth is unknown. Some materialize in days, weeks or months. Others take millions of years. Most diamonds are hundreds of millions of years old, with many dating back 1 to 3 billion years. That's because diamond growth isn't always a continuous process. A diamond might start to grow, then experience an interruption because of a change in temperature or pressure. It could sit for hundreds or millions of years before growth resumes.
How old are diamonds? A diamond can't be dated to find out its precise age, but inclusions of other minerals can suggest an estimate. Diamond inclusions that contain specific elements like potassium can be subjected to radioactive dating.
As the diamonds form deep in the earth, they withstand exposure to all sorts of gasses, minerals, and other materials surrounding the ore. Although the budding gem hardens to an unbreakable state, anything it touches during this phase can impact its color. Most diamonds appear white, but upon further inspection, have a yellowish tint or a light, almost imperceptible shade of brown. This doesn't mean that there is anything "wrong" with the diamonds, just that they're unique in their beauty.
Diamond Formation
So how do diamonds form? There are four main ways. We'll break them all down below, but you can also use the image just below this paragraph for an illustration of each point.
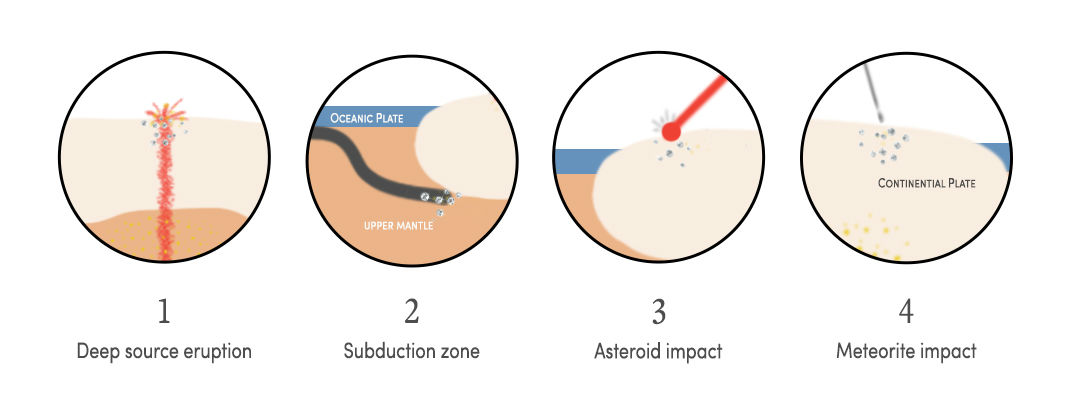
Diamond formation in the Earth's mantle
Diamond formation in subduction zones
Diamond formation at impact sites
Diamond formation in space
Most diamonds are found in commercial mines, but they were actually formed inside the Earth's mantle, about 150 kilometers below the Earth's crust. Diamonds are created in something called a "diamond stability zone" in the upper mantle, a high-pressure region that has a temperature of over 1,000 degrees Celsius. Diamonds are continually forming and growing there, just as they have been for billions of years. The gems are brought to the surface of the Earth during a seismic event like a volcanic eruption, embedded in large chunks of rocks called xenoliths.
Diamonds are also thought to form in "subduction zones," a.k.a. places where the tectonic plates that are constantly, gradually moving beneath the earth's surface come together, causing one plate to move underneath the other. Some studies suggest that subducted seawater is involved in the formation of diamonds in these zones; others have found these diamonds contain tiny bits of oceanic crust.
Intriguingly, some diamonds are believed to result from asteroid strikes. Whenever an asteroid hits the earth's surface, extremely high temperature and pressure is produced. Diamonds have been found in meteor craters in Arizona and Siberia, Russia, where excavators at the Popigai Crater have come across diamonds up to 13 millimeters in size.
Talk about space rocks. Researchers at NASA have found extremely small pieces of diamonds in extraterrestrial bodies. These have formed either in outer space or in the mantle of other planets.
Lab-Made Diamonds
Technology marches on, even when it comes to diamonds. Diamonds now can be made in a lab, and they're growing in popularity. Lab-grown diamonds will have the same look and feel of a natural diamond, because the growing process recreates the carbon-atom structures formed by Mother Nature.
When growing diamonds in a lab, technicians place acid into a heat and pressure chamber, replicating the natural growth process. The diamond crystallizes and matures within six to 10 weeks. The diamond is then polished and graded.
Lab-made diamonds are often called "synthetic," but that doesn't mean they're fake. They're identical to diamonds that are mined; they just didn't come from the earth. In fact, if you use any of the tests to find out if a diamond is real, a lab-made diamond will pass. Because it is a diamond. Learn how lab diamonds are created.
Diamond Chemical Structure and Strength of Diamonds
The chemical formula of a diamond is C, the same as the MVP of all elements, carbon. However, graphite and soot are also made of pure carbon atoms. So how do you differentiate between soot, graphite, and diamond? It has to do with the chemical structure of a diamond.
Carbon atoms can be arranged in many different physical forms, which are called allotropes. In a diamond, the carbon atoms are all covalently bonded to one another. They produce a three-dimensional network solid, a strong allotrope that differs from those of graphite and soot, which are more relatable to chicken wire.
How strong are diamonds compared to other gems? As the most concentrated form of pure carbon in the natural world, diamonds are the strongest mineral known on earth. They sit at the top of the Mohs hardness scale, which measures the ability of one mineral to scratch another visibly.
FAQs
Are diamonds made from coal?
How old are diamonds?
Is diamond the strongest mineral on earth?
Are lab diamonds as strong as natural diamonds?









